DEVELOPMENT PIPELINE
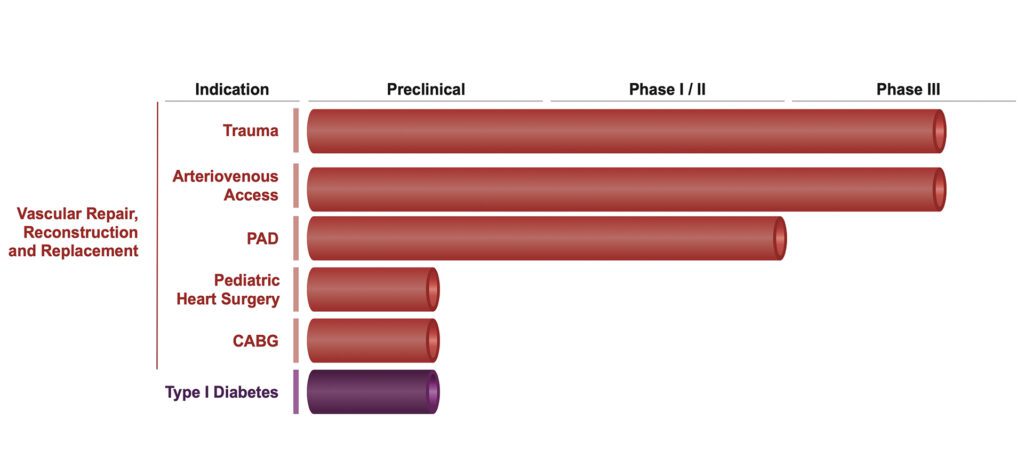
Vascular Access Clinical Program
V001 is an open-label, single treatment pilot study conducted in Poland of the HAV in patients with ESRD receiving hemodialysis who were not candidates for fistula. A total of 40 patients received an HAV and began hemodialysis between 4 to 8 weeks after implantation. The primary safety goal of the study was to evaluate the safety and tolerability of the HAV in ESRD patients undergoing routine dialysis; the primary efficacy goal was to determine the primary, primary assisted and secondary patency rates of the HAV at Month 6. Patients were followed for safety and efficacy for 24 months post-HAV implantation. Patients who had a patent HAV and who were continuing dialysis at Month 24 are continuing to be assessed in long-term follow up for patient and HAV status through 10 years post-implantation.
Results of the clinical study have been published in Lawson et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. 2016 14;387(10032):2026-34.
In addition, results that show remodeling of the HAV to become more similar to the native vasculature are published in Kirkton et al. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med. 2019 Mar 27;11(485):eaau6934.
Details of the study can be found at ClinicalTrials.gov using the Study Identifier NCT01744418.
V003 is an open-label, single treatment arm Phase 1 study conducted in the United States of the HAV in patients with ESRD receiving hemodialysis who were not candidates for fistula. Overall, a total of 20 patients were implanted with an HAV as a vascular access for hemodialysis and followed for safety and efficacy for 24 months post-HAV implantation. The primary safety goal was to evaluate the safety and tolerability of the HAV in ESRD patients who were on or were about to start hemodialysis and required an arteriovenous (AV) graft for dialysis access. The primary efficacy goal was to determine the primary, primary assisted, and secondary patency rates of the HAV at 6 months.
Results of the clinical study have been published in Lawson et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. 2016 14;387(10032):2026-34.
In addition, results that show remodeling of the HAV to become more similar to the native vasculature are published in Kirkton et al. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med. 2019 Mar 27;11(485):eaau6934.
Details of the study can be found at ClinicalTrials.gov using the Study Identifier NCT01840956.
V006 is a Phase 3, randomized, two-arm, comparative study conducted in the United States, Germany, Poland, Portugal, United Kingdom (UK) and Israel of the HAV in patients with ESRD who were targeted for implantation of an AV graft for hemodialysis. The study was designed to compare HAV with two common, commercially available ePTFE grafts: Gore® PROPATEN® Vascular Graft or Bard® Impra® Vascular Graft. Eligible patients were randomized to either HAV or ePTFE in a 1:1 manner. Overall, 355 patients were enrolled. All patients are followed through 24 months post-implantation for safety and efficacy. After 24 months, patients with a patent study conduit continue to be followed for up to 5 years post-implantation at routine study visits. The primary goal of the study is to compare the secondary patency of the HAV with that of the ePTFE graft when used as a conduit for hemodialysis. A secondary efficacy objective is to compare the primary patency of the HAV with ePTFE. A secondary safety objective is to compare the rate of access-related infections for the HAV with ePTFE. Other secondary efficacy objectives are to compare rates of interventions, primary assisted patency, and efficiency of dialysis between the HAV and ePTFE and to describe the remodeling of explanted samples from HAV and ePTFE. Other safety objectives were to compare the safety and tolerability of the HAV with ePTFE and to compare relative rates of true aneurysm and pseudoaneurysm formation.
Details of the study can be found at ClinicalTrials.gov using the Study Identifier NCT02644941 as well as in the EU Clinical Trials Register using EudraCT Number.
This is a Phase 3, open-label, randomized, two-arm, comparative study conducted in the United States of the HAV in patients with ESRD receiving hemodialysis. Patients are randomized to receive either the HAV for vascular access or an autogenous arteriovenous fistula. Patients are followed for 24 months after creation of the study access (either HAV or fistula) at routine study visits. After 24 months, patients in the fistula group with a fistula that is still patent will be followed (while it remains patent) for up to 5 years. After 24 months, all HAV subjects will be followed, regardless of patency, for 5 years. The primary goals of the study are to compare the ability of the HAV with that of autogenous arteriovenous fistula to support functional hemodialysis. Key secondary efficacy objectives are to compare time to loss of secondary patency (abandonment) of the HAV with that of autogenous arteriovenous fistula when all subjects have reached 12 months post-study access creation, and to compare the rates of hemodialysis access-related interventions between the HAV and autogenous arteriovenous fistula treatment groups over 12 months post-study access creation. The key secondary safety objective is to compare the rates of hemodialysis access-related infections between the HAV and autogenous arteriovenous fistula treatment groups over 12 months post-study access creation, irrespective of study access abandonment.
This study is currently enrolling as of December 2020. Details of the study can be found at ClinicalTrials.gov using the Study Identifier NCT03183245.
This is an open-label, single-arm Phase 2 study conducted in Poland of the HAV in patients with ESRD who are not candidates for autogenous arteriovenous fistula. The purpose of this study is to evaluate HAV that were manufactured on Humacyte’s commercial LUNA platform. Patients will have the HAV implanted in the forearm or upper arm using standard vascular surgical techniques. Subjects will then be followed for safety and efficacy assessments through 12 months, then for up to 36 months (3 years) while the HAV remains patent. The primary goal of the study is to evaluate the safety, efficacy, and immunogenicity of HAVs manufactured using the commercial manufacturing system (LUNA), and the secondary goal is to evaluate the long-term safety and efficacy of the HAV.
Details of the study can be found at ClinicalTrials.gov using the Study Identifier NCT04135417 as well as in the EU Clinical Trials Register using EudraCT Number 2018-003570-26.
Vascular Trauma Clinical Program
V005 is a non-randomized, open-label Phase 2/3 pivotal study conducted in the United States, Poland, and Israel of the HAV in patients with life- or limb-threatening vascular trauma. In this study, patients with life- or limb-threatening vascular trauma, in either the extremities or the torso, will be implanted with a Humacyte HAV as an interposition replacement or bypass using standard vascular surgical techniques. The primary efficacy goal is to assess the primary patency of the HAV at 30 days in vascular trauma patients undergoing surgery for vascular replacement or reconstruction. The primary safety goal is to evaluate the safety and tolerability of the HAV in vascular trauma patients undergoing surgery for vascular replacement or reconstruction. The secondary efficacy goals are to determine the primary patency of the HAV at 15 days; determine the primary, primary assisted, and secondary patency of the HAV at 6, 12, 24, and 36 months; determine the rates of interventions needed to maintain / restore patency in the HAV; determine the rate of limb salvage in the limb cohort; determine patient survival; determine infection rates of the HAV; evaluate remodeling of the HAV; and assess post-traumatic injury patient lifestyle. The secondary safety goals are to determine mechanical stability of the HAV based on freedom from aneurysmal degeneration, anastomotic bleeding or spontaneous rupture, infection, or significant stenosis; and determine the durability of the HAV repair in terms of freedom from HAV removal or replacement.
V005 is currently enrolling as of December 2020. Details of the study can be found at ClinicalTrials.gov using the Study Identifier.
Peripheral Arterial Disease (PAD) Clinical Program
V002 is an open-label, single treatment pilot study conducted in Poland of the HAV in patients with symptomatic PAD and claudication, requiring above-knee peripheral bypass surgery. Patients were implanted with an HAV using standard vascular surgical techniques. Safety and efficacy of the HAV was then followed for 2 years. Efficacy assessments evaluated patency, interventions on the HAV, and disease symptoms. For patients with a patent HAV, safety and efficacy assessments are ongoing through 10 years post-implantation. The primary safety goal was to evaluate the safety and tolerability of the HAV in PAD patients undergoing above-knee femoro-popliteal bypass grafting, and the primary efficacy goal was to determine the primary, primary assisted and secondary patency rates of the HAV over 24 months. Long-term follow up assessments include patency, disease symptoms, ankle-brachial index measuremenets, HAV interventions and complications, and HAV structural integrity.
Results of the clinical study have been published in: Gutowski et al. Arterial reconstruction with human bioengineered acellular blood vessels in patients with peripheral arterial disease. J Vasc Surg. 2020 Oct;72(4):1247-1258.
Details of the study can be found at ClinicalTrials.gov using the Study Identifier NCT01872208.
V002 is an open-label, single treatment pilot study conducted in Poland of the HAV in patients with symptomatic PAD and claudication, requiring above-knee peripheral bypass surgery. Patients were implanted with an HAV using standard vascular surgical techniques. Safety and efficacy of the HAV was then followed for 2 years. Efficacy assessments evaluated patency, interventions on the HAV, and disease symptoms. For patients with a patent HAV, safety and efficacy assessments are ongoing through 10 years post-implantation. The primary safety goal was to evaluate the safety and tolerability of the HAV in PAD patients undergoing above-knee femoro-popliteal bypass grafting, and the primary efficacy goal was to determine the primary, primary assisted and secondary patency rates of the HAV over 24 months. Long-term follow up assessments include patency, disease symptoms, ankle-brachial index measuremenets, HAV interventions and complications, and HAV structural integrity.
Results of the clinical study have been published in: Gutowski et al. Arterial reconstruction with human bioengineered acellular blood vessels in patients with peripheral arterial disease. J Vasc Surg. 2020 Oct;72(4):1247-1258.
Details of the study can be found at ClinicalTrials.gov using the Study Identifier NCT01872208.





