
Our Bioengineering
Technology Process
We’ve pioneered a scalable bioengineering process
to make tissue repair and replacement more readily accessible to a broader patient population.
Our first-in-class bioengineering technology and manufacturing process aims to transform human cells into universally implantable, regenerative tissues and advanced organ systems, for use in the treatment of injury and disease, across a wide range of clinical needs.
The Process
Cell Transfer
Human vascular cells from our cell banks are expanded and seeded onto a biodegradable scaffold made of polymer mesh to create tissues in different diameters and lengths. The cells proliferate and build an extracellular matrix (ECM).
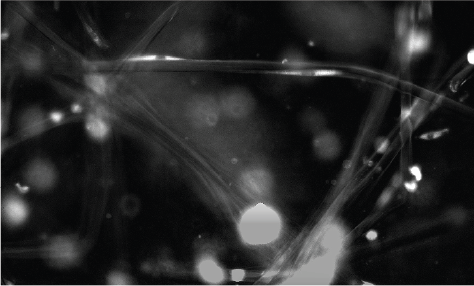
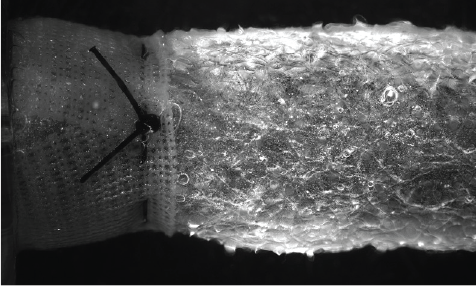
ATEV Formation
While in our proprietary bioreactor system, the cells are fed growth media and subjected to biomechanical stresses. The cells respond and align appropriately in order to generate tissue.
Over time, the polymer mesh degrades leaving cells and ECM.

Decellularization
Our proprietary decellularization process removes cells but retains key ECM proteins and structure to support biological activity.

The Goal
Universally implantable Acellular Tissue Engineered Vessels (ATEVTM), Advanced Organ Systems, and Advanced Tissue Constructs that have potential to address a wide range of clinical needs, and are all built on the foundational science of our Acellular Tissue Engineered Vessel platform.
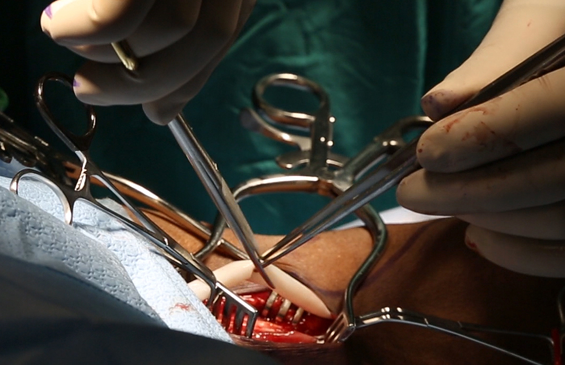
- Data on file.
